Understanding the immune response to the SARS-CoV-2 virus is a key step toward identifying a vaccine for COVID-19, and determining why COVID-19 can be mild for some people but life-threatening for others.
A person’s immune response is determined in part by a large number of protein molecules on the surface of cells. Many of these proteins are only found on a specific type of cell; B-cell receptor (BCR) proteins are only found on the surface of B-cells, and T-cell receptor (TCR) proteins are only found on the surface T-cells, where B-cells and T-cells are specific types of immune system cells. Others, like killer-cell immunoglobulin-like receptor (KIR) proteins, are found on the surfaces of natural killer (NK) cells and so-called killer T-cells. Finally, human leukocyte antigen (HLA) proteins are found on the surface of all cells except red-blood cells.
HLA proteins are like windows into the inner workings of a cell. These proteins bind peptides, short pieces of the many proteins found inside a cell, so that they can be inspected by T-cells. A T-cell uses the TCR to inspect the complex of an HLA molecule and its bound peptide on the cell surface. TCRs only recognize peptides that are foreign -- derived from a pathogen or a cell that is malfunctioning, like a cancer cell. When the TCR recognizes the complex of an HLA molecule binding a foreign peptide, it becomes activated, which is how the immune system becomes ‘aware’ of the presence a foreign protein, a sign of a likely infection. Activated T-cells can kill infected cells, or activate B-cells, which produce antibodies in response to an infection.
Some KIR proteins on NK cells and killer T-cells interact with HLA molecules. The absence of an HLA molecule on the surface of a cell can indicate a likely infection, in which case the NK cell or killer T-cell kills the cell.
These interactions are shown in the figure below, which outlines the process of SARS-CoV-2 infection of epithelial cells (the cells lining the surfaces of the respiratory system) and the subsequent immune response. In the figure, SARS-CoV-2 particles bind to ACE2 receptor proteins, which allow the virus to enter the cell.
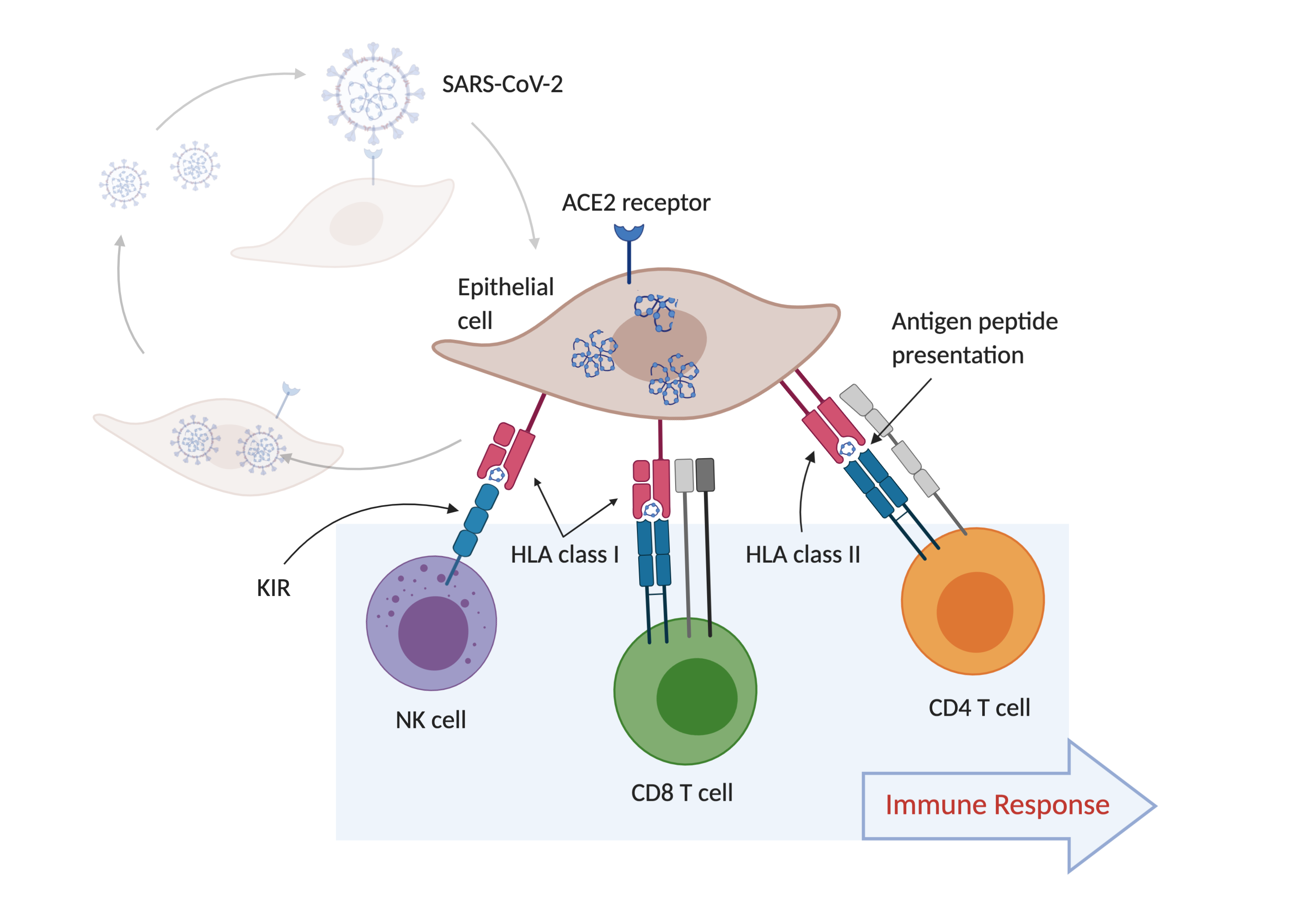
Variation in the amino-acid sequence of these BCR, TCR, KIR and HLA proteins is the key to making this system work. Amino-acid sequence variation in BCR and TCR molecules is generated within each person, so that a single person can have hundreds of thousands of different BCR and TCR protein molecules, which can recognize millions of foreign peptides, but the peptide sequence variation in HLA and KIR molecules is generated in human populations.
Each person has about 12-14 HLA proteins and 14-24 KIR proteins, half of which they inherit from each of their parents; the amino-acid sequence of these proteins does not change over a person’s life, but they sometimes change when genes are transmitted from parents to offspring. There are over 1100 versions (alleles) of the KIR genes, and almost 27,000 alleles of the HLA genes, in the human population.
Each of these alleles can have an effect on the larger immune system, both in the way that it interacts with the other receptor proteins, but also, in the case of HLA, in the number and kind of foreign peptides that can be bound. A given HLA allele can only bind a small subset of all the possible peptides that can be found in a cell. Because of this, specific HLA alleles are associated with protection or susceptibility to specific infectious diseases. In addition, some HLA alleles are only found in certain populationsor ethnic groups, or are much more common in some populations or ethnic groups than in others.
Our hypothesis in forming this consortium is that variation in a person’s HLA type, the collection of the 12-14 HLA proteins made by that person, has a direct effect on which of the peptides made from the proteins of the SARS-CoV-2 virus can be presented to and recognized by T-cells. This would mean that the immune systems of people with specific HLA types may be better at detecting the presence of the virus than others, and thereby better or worse at mounting an immune response and generating antibodies. Variation in the set of KIR genes that a person inherits, or the set of TCR and BCRs made by their immune systems, may have additional effects on the immune response.
The goal of the HLA|COVID-19 Consortium is to aggregate HLA type data, along with KIR type and other immune receptor types, collected for COVID-19 patients in multiple studies from around the world, to understand how variation in these immune protein molecules impacts the immune response to the disease. This knowledge will allow us to make predictions, based on a person’s immune molecule type, about a given person’s risk of contracting COVID-19 and the potential severity of the disease. In addition, direct knowledge of a person’s HLA type may allow the application of SARS-CoV-2 vaccines specifically targeted to work with a particular HLA type.